"Well, I'm doing a PhD," I usually reply. At which point the conversation usually turns to chemistry and someone says. "So it must be exciting to work with all those chemical and tubes and stuff," to which I have to reply. "Erm, well actualy I work at a computer all day. Haven't been in an experimental lab for a couple of years really." My companion's eyes glaze over, the conversation lost once more.
So here I am, about to explain what it is that I do without the use of equations etc which tend to scare some arts students rather. What I (and my supervisor) are currently trying to find out is how fast does hydrogen travel through palladium. The more astute of you will at this point be pointing out that palladium is a metal and metals are hard - you can't get gases to go through them. But infact you can - so there!
A hydrogen atom is very small (just a proton and an electron) and is about 100x smaller than your average palladium atom. This has some rather unfortunate consequences - the most unfortunate being that in order to describe what the hydrogen atom is doing properly you have to use quantum mechanics.
"Nooooooooo!" I hear you cry. "I've never understood it." Well me neither, but here goes. In quantum mechanics everything is described by a wavefunction. A wavefunction is just something that gives you a number at any point in space (like a sine function for instance). If you square the wavefunction you get the probability of finding the particle at that point. You can also work out useful things like the energy and velocity of your particle from its wavefunction, so it's a useful thing to have.
Unfortunately it's a bit of a pain to find out, and that's what I've been doing. I'm writing a computer program to work out the wavefunction of a hydrogen atom in palladium and from that to work out the speed of diffusion.
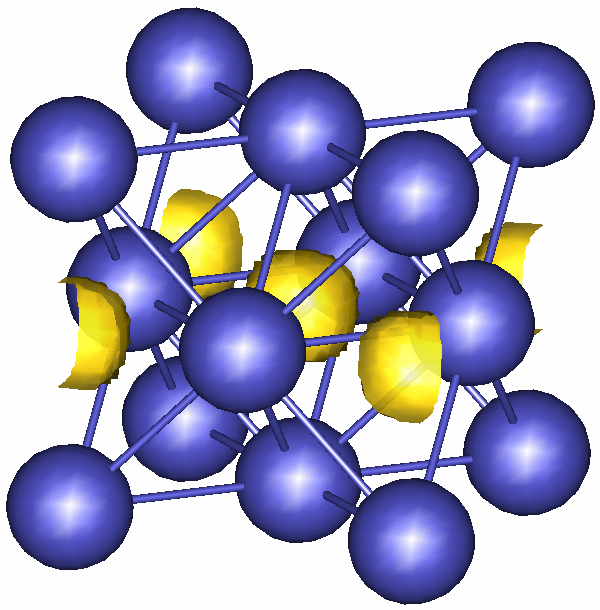
Right, enough of all that, and here's a picture. The big dark grey circles are palladium atoms. Imagine that the cube of palladium atoms extends in all directions, up, down, sideways and front and back and you'll be sitting in the middle of a palladium lattice. The length of this box is about 1 / 2,500,000 of a millimeter (so quite small).

The yellow bits are where the square of the hydrogen atom wavefunction is quite big (i.e. they show the areas where you are most likely to find a hydrogen atom in a palladium lattice). You may (or may not) be able to see that they sit in the middle of octahedra made up of 6 palladium atoms.
You may well ask whether any of this is of any practical use and unfortunately funding bodies seem rather interested in this as well. Fortunately palladium hydride is quite a useful thing. Since hydrogen will dissolve in it, it can be used as a storage device for hydrogen. Palladium films can be used to filter hydrogen gas out of a mixture of gases, since only hydrogen can pass through it quickly. Palladium is also a good catalyst for many reactions, some of which include hydrogen. A better understanding of the system should allow people more practical than myself to put palladium hydride to good use.
So, there you go, a brief description of what I get up to at work. I hope you enjoyed it and that it at least made some sense.
Matthew